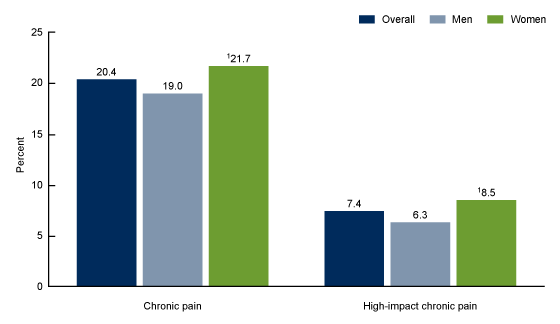
Chronic Pain - An Unsolved Health Problem
Chronic pain remains a widespread, incapacitating health issue with significant physical and emotional impact. According to CDC (Figure 1) about 1 in 5 adults suffer from chronic pain and about 1 in 15 adults suffer from high impact chronic pain, which can significantly affect the quality of life. Chronic pain often leads to depression and to substantial, long-term anatomical and physiological changes in the brain. There is a wide spectrum of chronic pain syndromes resulting from injuries, inflammation (e.g. rheumatoid arthritis), disease (e.g. diabetic neuropathy), genetic factors or unknown causes. Despite extensive and expensive research efforts into the mechanisms of chronic pain, treatment is currently limited to palliative rather than curative solutions.
In addition to the pain itself, the connection between chronic pain and depression has long been known. This is in fact a 2-way interaction mediated by changes in neurotransmitter and hormonal levels. Thus chronic pain affects quality of life on multiple levels.

Inadequacy of Current Chronic Pain Treatments
The inadequacy of models and hypotheses in chronic pain research translates into inadequate treatments. Despite enormous expenses through government grants and pharmaceutical research, no new therapies have emerged. In addition to highly addictive opioids, used to treat pain since ancient times, chronic pain treatments continue to rely almost exclusively on the off-label use of drugs designed for other conditions: anti-depressants, anticonvulsants, antipsychotics and benzodiazepines. These drugs have a highly addictive potential by concurrently influencing the reward system in the brain, as well as a long list of serious adverse effects.
In addition, due to natural physiological mechanisms, the long-term use of such drugs unavoidably leads to receptor desensitization and/or downregulation, requiring increasingly higher drug doses to maintain the same level of analgesia (habituation). Since most of these drugs also have significant adverse effects which limit the amount of drug that can be administered, any of these treatments will eventually reach the maximal dose and then fail to continue providing an adequate level of analgesia.
About the Mechanisms of Pain
Acute pain has evolved from the first monocellular organisms to detect and warn against external threats. Thermal, pressure, inflammatory, chemical or pain stimuli are detected by a variety of receptors in the skin or in the internal organs, and are converted into electrical signals which are transmitted to the brain by a sequence of specialized neurons (Figure 2). A major role in this transmission is played by the spinal cord, which performs some preliminary processing of the pain signals in its dorsal region before sending these signals to the brain.
In contrast, chronic pain, which continues to stimulate pain circuits long after the initial stimulus has ceased, does not have a useful role and is perceived as an annoying and, often, as an incapacitating condition. The borderline between acute and chronic pain has been arbitrarily defined by most health practitioners as 6 months, but a 3 months demarcation line is more appropriate based on physiological mechanisms, as further explained here.
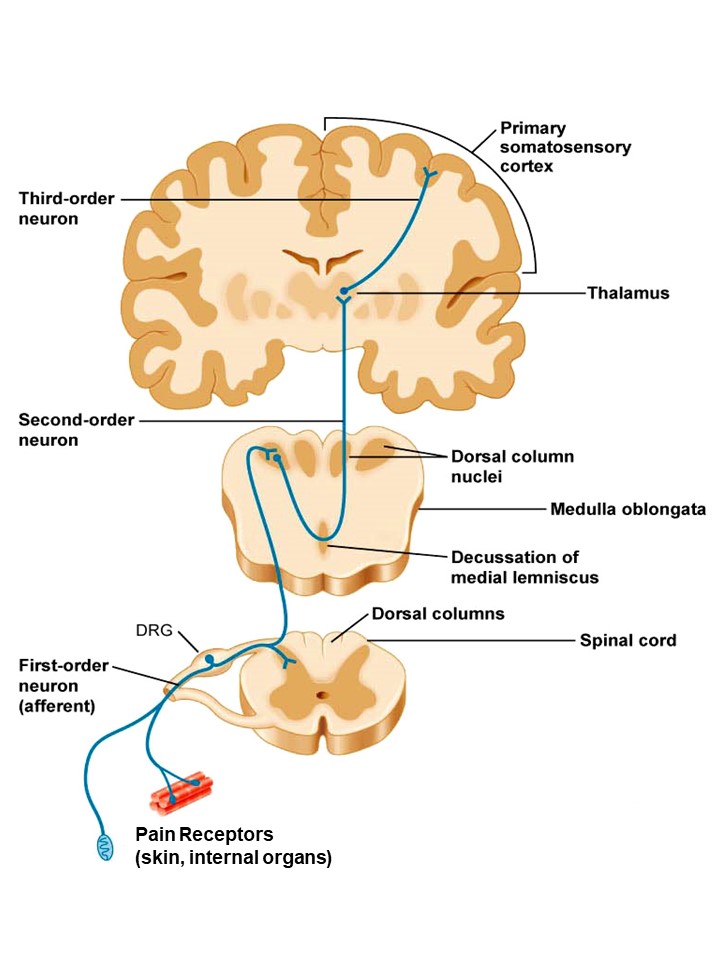
Figure 2. Diagram of pain transmission from sensory pain receptors to the brain, via the spinal cord.
Pain Circuits Are Dynamic
Over the past 20 years, neuroscience has been revolutionized by a series of exciting discoveries. One of the most important new concepts is that the nervous system is not static as previously thought. Neuronal stem cells are present in many regions of the central nervous system, including the spinal cord, with the ability to generate new neurons. Most of the time, these neuronal stem cells are quiet, but serious injuries or inflammation that overstimulate the pain circuits will induce them to divide to generate new neurons. Due to complex cellular processes, such newly generated immature neurons have a higher excitability and are thus easier to stimulate until they fully mature into adult, less excitable neurons.
We have found that these newly generated, hyperactive neurons tend to migrate and accumulate preferentially in the dorsal region of the spinal cord, the same region responsible for pain processing (Figure 3). There is a strong correlation between the increased level of pain sensitivity and the excess of immature hyperexcitable neurons present in the spinal cord area responsible for pain processing. This explains why pain sensitivity is increased for an extended duration of time after an injury serious enough to induce spinal cord inflammation and the activation of stem cell proliferation.
The production of new neurons from these activated stem cells reaches its peak around week 6 after injury, as shown in Figure 3. These immature hyperactive neurons mature in approximately 6 weeks, when they become less active, therefore it normally takes a total of around 3 months for the pain to subside after its trigger by a serious injury. A pain lasting significantly longer than 3 months after the initial injury indicates that there are additional factors involved which lead to chronic pain. Based on these physiological processes, chronic pain can be defined as lasting for more than 3 months after the cause of injury was removed.
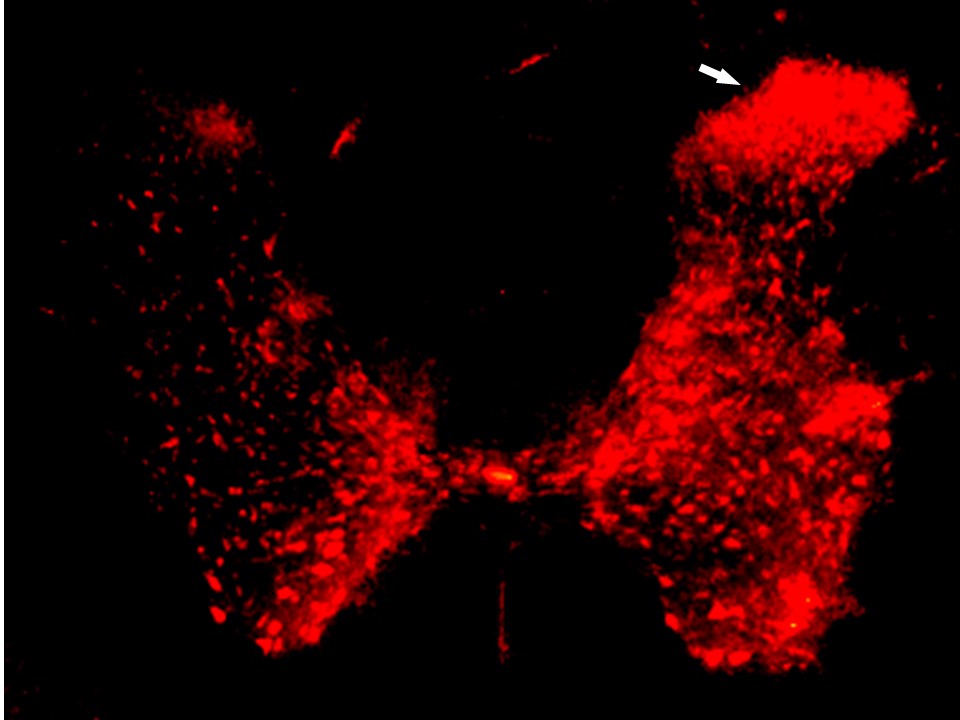
Why Does Pain Become Chronic?
There are many factors, either psychological or pathophysiological, that can lead to chronic pain. However, many such factors can be included in a simple explanation. If the increased excitability of immature neurons can lead to an increase in pain sensitivity, while their subsequent maturation can eliminate that pain, it is logical that factors that interfere with the normal neuronal maturation process and maintain a large number of immature hyperexcitable neurons extend the duration of pain beyond the 3 months expected under normal conditions. Thus pain can be extended for years or even for life.
In some cases factors responsible for chronic pain involve a disease that maintains a chronic inflammatory environment (e.g. arthritis) or induces nerve degeneration (e.g.diabetic neuropathy). In many other cases however, the cause of chronic pain persistence long after the injury has healed remains unexplained. Another category includes chronic pain syndromes present without ever having an injury, such as fibromyalgia, persistent idiopathic facial pain (PIFP), joint hypermobility syndrome, Marfan syndrome, complex regional pain syndrome (CRPS), paroxismal extreme pain disorder (PEPD), and others. Some groups, ranging from families to entire ethnic groups, are characterized by an increased pain sensitivity in otherwise perfectly healthy individuals. In these groups it is likely that genetic variations may be responsible for chronic pain and some of these variations have been already identified.
The Genetics of Chronic Pain
Over two dozen genes have been so far specifically linked to increased pain sensitivity, either in humans or in laboratory animal experiments. Each of these genes has different functions, which is confusing and makes a logical approach to treatment very difficult. However, a closer look shows that all these genes do have something in common: they are involved in one way or another in the process of neuronal differentiation, which controls the transformation of newly generated immature neurons into less active mature neurons. This striking coincidence between neuronal maturation and pain sensitivity suggests that gene variations that interfere with normal neuronal maturation are likely to drive an increase in pain sensitivity due to the hyperactivity of neurons maintained in an immature state for an extended time.
A New Model of Chronic Pain
According to the above observations, if a serious injury triggers the production of a wave of immature neurons from stem cells in the spinal cord, in the average, normal individual these hyperactive cells will mature within 3 months and pain sensitivity will be restored to normal. However, in an individual with gene variations that interfere with, or slow down normal neuronal maturation, those newly generated neurons will remain for an extended period of time in an immature hyperactive state. These immature neurons will continuously stimulate nerve circuits responsible for pain processing even in the absence of a painful stimulus, which is perceived as chronic pain.
This model can also explain chronic pain syndromes present in the absence of any injury. Waves of immature neurons are generated from neural stem cells at birth and the first years of life. Normally, some of these neurons mature while the rest die off. If genetic variations interfere with neuronal maturation, many of these immature neurons may survive into adulthood, continuing to stimulate pain circuits even in the absence of an injury.